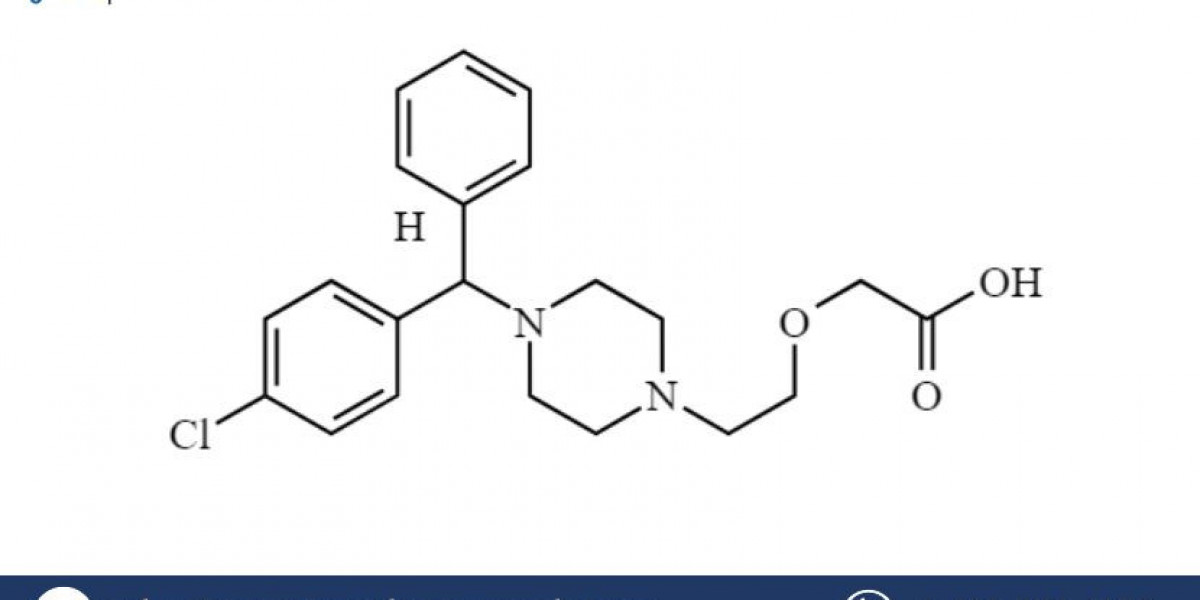
Levocetirizine (brand name Xyzal) is an antihistamine commonly used to treat allergy symptoms such as runny nose, sneezing, and itchy or watery eyes. It is also used to alleviate symptoms of allergic rhinitis and chronic urticaria (hives). Due to the growing prevalence of allergic diseases globally, the demand for antihistamines like Levocetirizine is increasing. Establishing a Levocetirizine manufacturing plant provides a lucrative opportunity in the pharmaceutical industry. This Levocetirizine (Xyzal) Manufacturing Plant Project Report details the steps and considerations for setting up a manufacturing facility for Levocetirizine, including market demand, raw material procurement, production processes, equipment requirements, regulatory compliance, and safety standards.
Overview of Levocetirizine (Xyzal)
Levocetirizine is a second-generation antihistamine that works by blocking the action of histamine in the body, reducing allergy symptoms. It is a more potent form of cetirizine, the active ingredient in Zyrtec, with fewer sedative effects, making it ideal for daytime use. Levocetirizine is available in both tablet and syrup form and is typically prescribed for seasonal and perennial allergic rhinitis and chronic urticaria.
The drug has a high safety profile, making it suitable for long-term use in treating allergic conditions. Due to its effective action and minimal side effects, it is one of the most commonly prescribed antihistamines worldwide.
Raw Materials for Levocetirizine Production
The production of Levocetirizine requires various raw materials, including:
- Levocetirizine Hydrochloride: The primary active ingredient.
- Excipients: Substances like lactose, cellulose, and magnesium stearate used in tablet formulations.
- Solvents and Reagents: Acetone, ethanol, and other chemicals for synthesis and purification.
- Packaging Materials: Bottles, blister packs, and labels for final product packaging.
Get a Free Sample Report with Table of Contents@
Production Process
The manufacturing of Levocetirizine involves several stages, including:
Synthesis of Levocetirizine:
The chemical synthesis of Levocetirizine starts with the preparation of its precursor, which undergoes various chemical reactions to form the active pharmaceutical ingredient (API).Purification:
The crude Levocetirizine is purified using filtration and crystallisation methods to remove impurities and achieve the required pharmaceutical-grade purity.Formulation:
The purified Levocetirizine is then mixed with excipients to form tablets or syrups. The formulation process ensures the correct dosage and stability of the product.Quality Control and Testing:
After production, the product undergoes rigorous quality control checks to ensure it meets safety and efficacy standards. This includes testing for potency, purity, dissolution rate, and microbiological contamination.Packaging:
The finished tablets or syrup are then packaged into appropriate containers (blister packs or bottles) with clear labels. The final packaging is designed to maintain the stability and safety of the product during storage and transport.
Key Equipment Required
To set up a Levocetirizine manufacturing plant, the following equipment will be needed:
- Reactors and Mixing Tanks: For chemical reactions and the blending of raw materials.
- Filtration and Purification Units: For removing impurities from the active ingredient.
- Drying Equipment: Such as spray dryers or tray dryers to remove moisture from the product.
- Tableting Machines: For tablet production, including compressing, coating, and packing.
- Liquid Filling Machines: For filling liquid formulations such as syrups.
- Packaging Equipment: For blister packing, bottle filling, and labeling.
Regulatory and Compliance Considerations
When establishing a Levocetirizine manufacturing plant, compliance with local and international regulatory standards is crucial. Key regulations include:
- Good Manufacturing Practices (GMP): Ensures the facility operates with the highest standards of hygiene, safety, and quality.
- FDA/EMA Approval: The manufacturing plant must be certified by regulatory bodies like the U.S. FDA or the European Medicines Agency (EMA) before commercial production.
- Environmental Regulations: Adherence to environmental safety standards to control chemical waste and emissions.
- Quality Control Regulations: Compliance with pharmacopoeia standards for raw materials, production processes, and final products.
Market Demand and Opportunities
The global market for antihistamines, including Levocetirizine, is growing, driven by increasing allergic conditions and the shift towards over-the-counter medications. With Levocetirizine being a popular choice for both seasonal and perennial allergies, the demand for its production is expected to remain strong.
Some of the key factors contributing to this growth include:
- Increasing Allergic Conditions: Rising pollution levels, climate change, and lifestyle factors contribute to more people suffering from allergies.
- Rising Healthcare Awareness: As people become more aware of their health, there is growing demand for effective over-the-counter medications.
- Expanding Pharmaceutical Market: The increasing focus on healthcare and the rise of chronic diseases in emerging markets create significant opportunities for Levocetirizine.
Cost Considerations and Financial Overview
The setup cost for a Levocetirizine manufacturing plant includes expenses for land acquisition, construction, machinery, raw materials, and licensing. Other factors such as staffing, regulatory compliance, and marketing costs also need to be considered. A detailed financial analysis, including the return on investment (ROI), payback period, and operating costs, is essential for understanding the long-term viability of the business.
FAQ
What is Levocetirizine used for?
Levocetirizine is used to treat allergy symptoms like sneezing, itching, and runny nose, as well as chronic hives (urticaria).What raw materials are required for manufacturing Levocetirizine?
Key raw materials include Levocetirizine hydrochloride, excipients like lactose and cellulose, solvents for synthesis, and packaging materials.What are the primary steps in Levocetirizine production?
Production involves chemical synthesis, purification, formulation (tablets or syrup), quality control, and packaging.What equipment is necessary for Levocetirizine manufacturing?
Essential equipment includes reactors, filtration units, tablet press machines, liquid filling machines, and packaging systems.What regulations must a Levocetirizine plant comply with?
The plant must adhere to Good Manufacturing Practices (GMP), FDA/EMA regulations, and environmental safety standards.How can I ensure the quality of Levocetirizine during production?
Regular quality control checks, including potency, purity, and stability testing, ensure the product meets safety and efficacy standards.Is the market for Levocetirizine growing?
Yes, the demand for Levocetirizine is expected to grow due to increasing allergic conditions and rising healthcare awareness globally.
Media Contact
Company Name: Claight Corporation
Contact Person: Lewis Fernandas, Corporate Sales Specialist — U.S.A.
Email: sales@expertmarketresearch.com
Toll Free Number: +1–415–325–5166 | +44–702–402–5790
Address: 30 North Gould Street, Sheridan, WY 82801, USA
Website: www.expertmarketresearch.com
Aus Site: https://www.expertmarketresearch.com.au