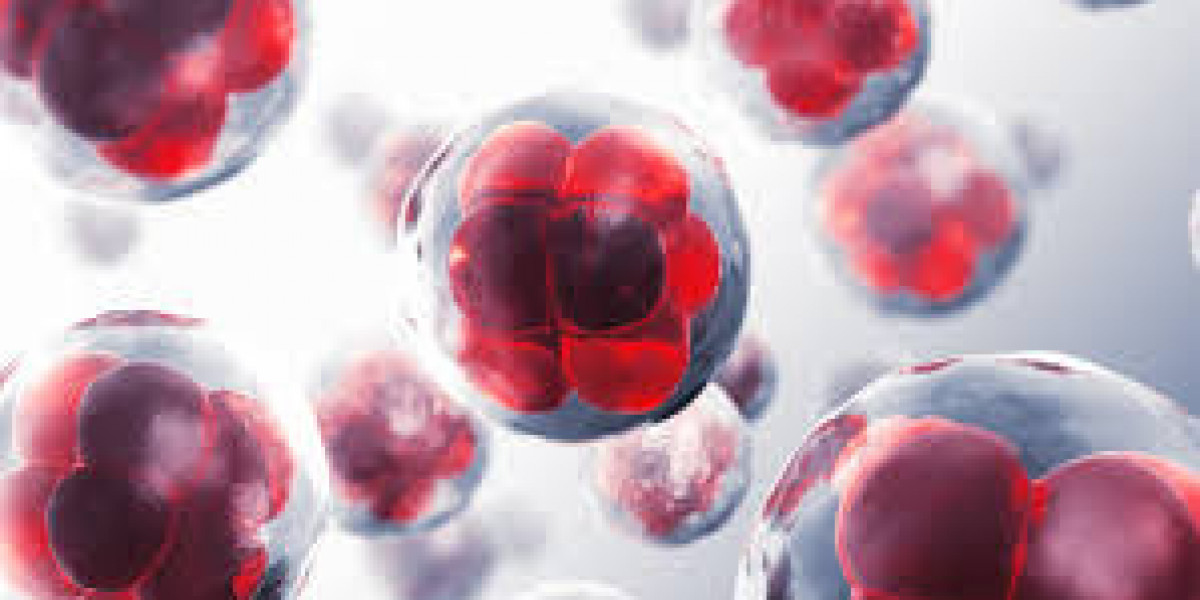
The field of autologous cell therapy market has witnessed profound disruptions in recent years, driven by significant advancements in medical research, technology, and healthcare solutions. Autologous cell therapy refers to a medical procedure in which a patient's own cells are utilized to treat or cure a condition, whether it be through regenerative medicine or immune therapies. This approach offers several advantages over traditional methods, including reduced risk of immune rejection and better integration with the patients biological system. However, the market is evolving rapidly, and numerous factors are contributing to its current disruptions.
Technological Advancements in Autologous Cell Therapy
The introduction of cutting-edge technologies such as CRISPR gene-editing tools and automated cell processing systems has significantly altered the landscape of autologous cell therapy. The ability to modify or enhance cells to meet specific therapeutic needs has opened new doors in the treatment of conditions like cancer, heart diseases, and autoimmune disorders. These technological strides have led to the creation of personalized therapies that offer tailored solutions, thus accelerating market growth.
Furthermore, advancements in cell isolation, culture techniques, and ex vivo expansion have played a pivotal role in enhancing the scalability and efficiency of autologous cell therapies. Previously, the processing of autologous cells was time-consuming and cumbersome, making it difficult to implement on a large scale. Now, with improvements in manufacturing and automation, therapies can be produced more quickly, driving down costs and increasing accessibility for patients across the globe.
The Role of Regulatory Changes and Market Expansion
A significant driver of disruption in the autologous cell therapy market is the evolving regulatory environment. Regulatory bodies such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) are gradually developing more precise frameworks to support the commercialization of advanced cell therapies. These changes are helping to streamline the approval process, making it easier for companies to bring new therapies to market.
The expanding acceptance of regenerative medicine is also contributing to the markets disruption. Conditions that were once considered untreatable are now being managed with autologous cell therapies. As research into stem cells, mesenchymal cells, and other regenerative cell types progresses, it is expected that more conditions will be addressed, resulting in broader market applications.
Rising Investment and Partnerships
With the increasing demand for autologous cell therapies, investment in this sector has skyrocketed. Pharmaceutical companies, biotech firms, and venture capitalists are pouring significant amounts of funding into research and development efforts. This surge in financial support is further accelerating the pace of innovation and bringing new therapies to market more quickly.
Additionally, collaborations between academic institutions and biotech companies have become more common, leading to breakthroughs in the design and implementation of autologous therapies. Strategic partnerships have enabled the pooling of resources, knowledge, and expertise, allowing for faster development cycles and improved patient outcomes.
Challenges in Manufacturing and Distribution
Despite the impressive advancements in autologous cell therapy, several challenges remain. One of the biggest hurdles is the complex and expensive manufacturing process. Autologous therapies require individualized treatment for each patient, which can lead to significant costs in production, especially when scaling up operations for larger patient populations.
Furthermore, ensuring the safe and efficient distribution of these therapies presents logistical challenges. The need to maintain cell viability during transport and storage adds another layer of complexity to the already intricate process. As a result, manufacturers and healthcare providers are looking for innovative ways to streamline these processes and reduce costs.
The Future of Autologous Cell Therapy
Looking forward, the future of autologous cell therapy is incredibly promising. Innovations in cell sourcing, gene editing, and automated production processes are expected to continue driving market growth. Moreover, the increasing demand for personalized medicine is likely to contribute to a shift in how healthcare providers approach patient treatment, with more individualized therapies becoming the standard.
In summary, the autologous cell therapy market is experiencing rapid disruption due to technological advancements, regulatory support, and an influx of investment. While challenges remain in terms of manufacturing and distribution, the potential for these therapies to revolutionize the healthcare landscape is undeniable. As research and development continue to progress, autologous cell therapies will likely become a cornerstone of modern medicine, offering hope to patients with a wide range of conditions.