The Global Spinal Muscular Atrophy Market is estimated to driven by Increased screening and early diagnosis
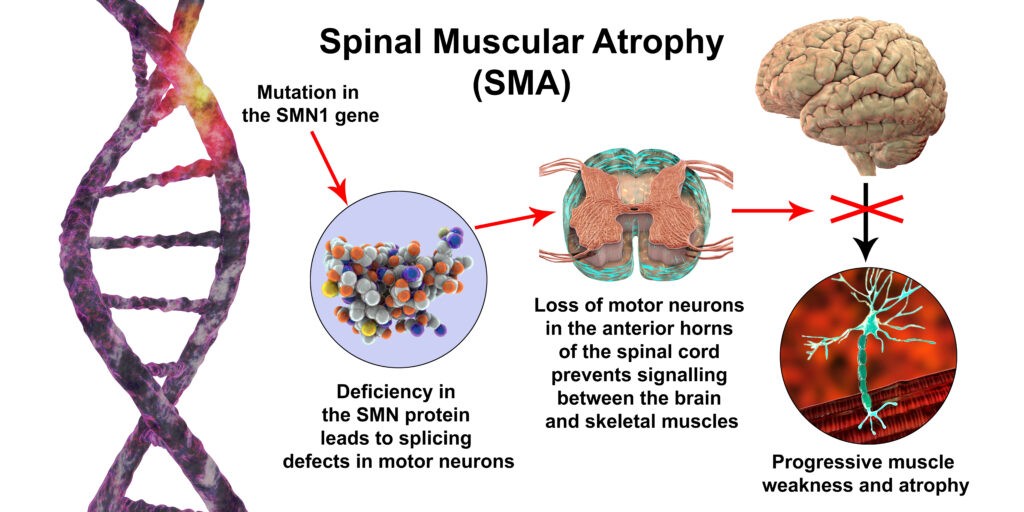
Spinal muscular atrophy (SMA) is a genetic disorder that affects the motor nerve cells in the spinal cord, resulting in weakness and wasting of muscles. SMA is caused by a mutation or deficiency of the survival motor neuron 1 (SMN1) gene. There are three types of SMA - type I being the most severe form that begins within the first 6 months of life. Treatment options for SMA include Nusinersen, onasemnogene abeparvovec, and risdiplam. Nusinersen is the first approved treatment for SMA, and works by modifying splicing of the SMN2 gene to increase levels of functional SMN protein. Onasemnogene abeparvovec is a gene therapy that provides a fully functional copy of the SMN1 gene. Risdiplam is an orally administered small molecule that increases SMN protein levels. These innovative therapies have improved clinical outcomes for SMA patients.
The Global Spinal Muscular Atrophy Market is estimated to be valued at US$6.5 billion in 2024 and is expected to exhibit a CAGR of 13% over the forecast period 2024-2031.
https://www.ukwebwire.com/sma-market-grows/