Gel electrophoresis and SDS-PAGE are two essential techniques used to analyze biomolecules like proteins and nucleic acids. Though they are related and often used together, they have distinct characteristics and serve different purposes depending on the scientific needs of researchers. Understanding the key differences between gel electrophoresis and SDS-PAGE is vital, especially for those utilizing advanced technologies like 2D Gels.
In this blog, We'll explore how gel electrophoresis differs from SDS-PAGE and how 2D Gels play a critical role in protein analysis, offered by Kendrick Labs, Inc.
What is Gel Electrophoresis?
Gel electrophoresis is a technique used to separate molecules based on their size, charge, or shape. The process involves placing samples, such as proteins or nucleic acids, into a gel matrix (either agarose or polyacrylamide), and applying an electric current. Molecules move through the gel at different rates depending on their properties, allowing for effective separation.
Key Features of Gel Electrophoresis:
Versatility: Gel electrophoresis can be used for a range of biomolecules, including DNA, RNA, and proteins. Agarose gel electrophoresis is often used for larger molecules like DNA, while polyacrylamide gel electrophoresis is better suited for proteins and smaller nucleic acids.
Basic Setup: Gel electrophoresis is a relatively simple technique. It involves loading samples into wells in the gel, applying an electric field, and letting the molecules migrate through the gel matrix. Smaller molecules move faster than larger ones.
Visualizing Results: After electrophoresis, the separated molecules can be visualized using dyes, stains, or other detection methods. This allows scientists to analyze molecular size and composition easily.
While gel electrophoresis offers an essential foundation for molecular separation, more advanced techniques like SDS-PAGE and 2D Gels provide additional precision and insight, particularly for protein analysis.
What is SDS-PAGE?
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) is a specialized form of gel electrophoresis used specifically for proteins. SDS, an anionic detergent, is used to denature proteins and give them a uniform negative charge. This eliminates any variability in the natural charge of the proteins, allowing them to be separated purely based on their size.
Key Features of SDS-PAGE:
Protein Separation by Size: Since SDS masks the natural charge of proteins and ensures they all carry a negative charge, the only factor affecting their migration through the gel is their size. Smaller proteins move faster through the polyacrylamide matrix, providing a clear size-based separation.
Denaturing Conditions: SDS-PAGE is performed under denaturing conditions, which means the proteins are unfolded and lose their natural structure. This makes SDS-PAGE an excellent tool for comparing protein sizes but limits its ability to study native protein structures or complexes.
Single-Dimensional Analysis: SDS-PAGE is a one-dimensional technique, meaning it separates proteins based on one characteristic—size. While it provides excellent resolution for size-based analysis, it lacks the ability to separate proteins based on charge or other characteristics. This is where 2D Gels come into play.
How Do 2D Gels Build on SDS-PAGE?
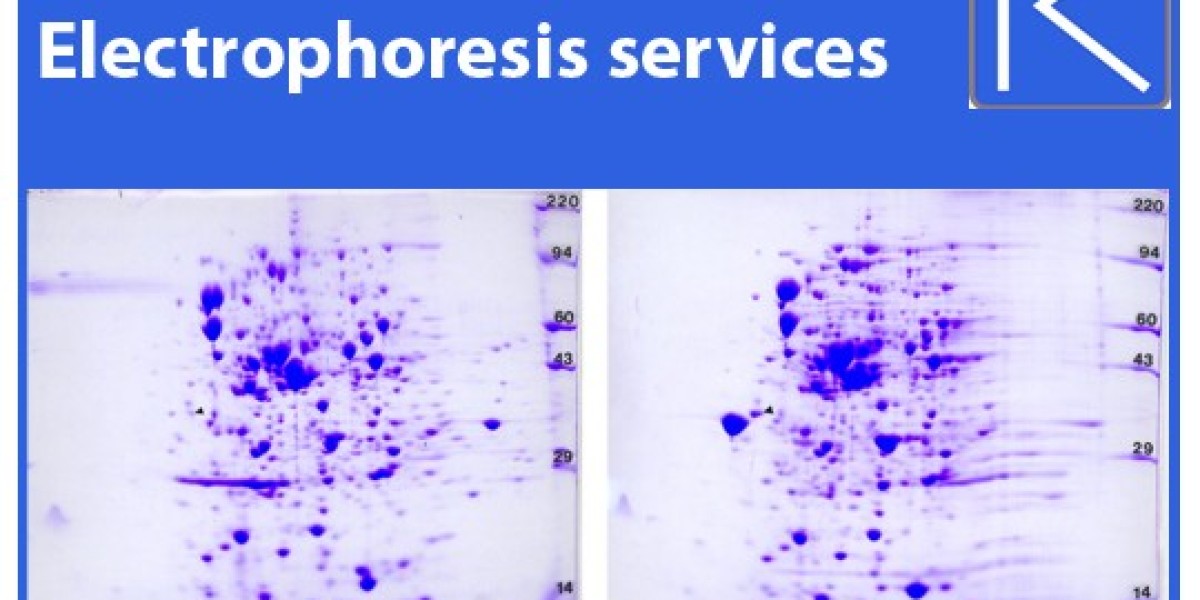
2D Gels, or two-dimensional gel electrophoresis, represent a more advanced technique that combines isoelectric focusing (IEF) with SDS-PAGE to separate proteins based on both their isoelectric point (pI) and molecular weight. This two-dimensional separation allows researchers to resolve complex protein mixtures with much higher resolution than one-dimensional SDS-PAGE alone.
Key Features of 2D Gels:
Dual Separation: 2D Gels first separate proteins based on their isoelectric point in the first dimension using IEF. Then, in the second dimension, SDS-PAGE is used to separate the proteins by size. This two-step process results in a highly detailed map of the protein sample.
Comprehensive Protein Analysis: 2D Gels allow scientists to detect protein isoforms, post-translational modifications, and other subtle variations that SDS-PAGE alone cannot reveal. This makes 2D Gels an invaluable tool for proteomics research.
Application in Complex Samples: For researchers analyzing complex biological samples, such as tissue extracts or cell lysates, 2D Gels provide unparalleled resolution and depth. By offering dual separation, 2D Gels can detect and resolve thousands of proteins in a single run.
Why Choose 2D Gels Over SDS-PAGE?
While SDS-PAGE is an excellent method for simple, size-based protein analysis, it has limitations when dealing with more complex samples. 2D Gels offer a much more comprehensive approach, separating proteins based on both size and charge. This is particularly important for researchers studying proteomics or looking for subtle changes in protein structure and function.
At Kendrick Labs, Inc., we specialize in 2D Gels and offer cutting-edge electrophoresis services for researchers across various scientific fields. Our expertise ensures that complex protein samples are separated with the highest possible resolution, allowing for accurate and detailed analysis.
Conclusion
While both gel electrophoresis and SDS-PAGE are essential techniques in molecular biology, 2D Gels offer a more advanced solution for researchers needing high-resolution protein analysis. 2D Gels combine the strengths of SDS-PAGE with isoelectric focusing, providing comprehensive protein separation that is unmatched by one-dimensional methods.
If you’re in need of advanced protein separation techniques, Kendrick Labs, Inc. offers top-tier 2D Gels services. Our expertise can help you uncover detailed insights into your protein samples, enabling groundbreaking research in proteomics and beyond. Contact us today to learn more about how 2D Gels can enhance your research.
Original Source: https://kendricklabs.blogspot.com/2024/09/how-does-gel-electrophoresis-differ.html